A photocatalytic ensemble HP-T@Au-Fe3O4: synergistic and balanced operation in Kumada and Heck coupling reactions†
Abstract
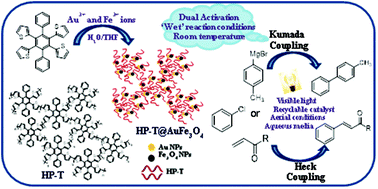
A supramolecular catalytic ensemble HP-T@Au-Fe3O4 supported by highly branched assemblies of hexaphenylbenzene (HPB) derivatives has been developed. The as-prepared HP-T@Au-Fe3O4 nanohybrid material serves as an efficient catalytic system to prepare biaryl derivatives through the Kumada cross-coupling reaction using aryl chlorides as one of the coupling partners under mild reaction conditions (visible light irradiation, aqueous media, aerial conditions, short reaction time). Through the cooperative effect of Au NPs and Fe3O4 NPs, dual activation of aryl chlorides for the generation of aryl radical intermediates is achieved. On the other hand, oligomeric assemblies contributed significantly to the enhancement of the reaction rate and yield of the product by facilitating the reductive elimination step. Different mechanistic studies confirm the involvement of Au NPs, Fe3O4 NPs and oligomeric assemblies in the synergistic and balanced operation of HP-T@Au-Fe3O4 nanohybrid materials in the efficient completion of the catalytic cycle of the Kumada coupling reaction. Being magnetic, the catalytic ensemble could be recycled for up to five catalytic cycles. The as-prepared supramolecular photocatalytic ensemble also works efficiently in Heck coupling reactions involving aryl chlorides and aryl iodides as the coupling partner.


 Please wait while we load your content...
Please wait while we load your content...