Rediscovering aminal chemistry: copper(ii) catalysed formation under mild conditions†
Abstract
Aminals, the N,N analogues of acetals, have been thoroughly explored in organic chemistry, with a particular focus on heteroaromatic aldehyde lithiation. Nevertheless, the existing methodologies for their formation typically employ harsh conditions limiting their usefulness. In this work, we present an efficient and mild methodology for the preparation of aminals from aromatic aldehydes, including furanic platforms. These mild conditions allowed ease of access to a plethora of aminals and as such we set out to explore previously unaccessible potential applications. By studying the stability of various aminals, we were able to develop a simple aldehyde protecting group based on a commercial diamine which is deprotected under mind conditions. We developed a protocol for the scavenging of genotoxic aldehydes by taking advantage of our methodology and a diamine resin, as well as early studies on the development of a stimuli-responsive release system using a salycil aldehyde derived aminal.
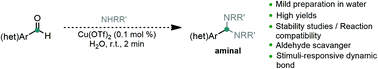


 Please wait while we load your content...
Please wait while we load your content...