NaCl-promoted phase transition and glycosidic bond cleavage under microwave heating for energy-efficient biorefinery of rice starch†
Abstract
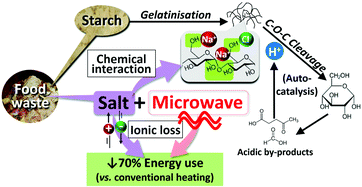
Sustainable utilisation of food waste in biorefineries is imperative to actualising a circular economy and alleviating the massive environmental burden. An efficient biorefinery system design necessitates comprehensive understanding of the impurity effects such as salt. This study scrutinised the hydrolysis of rice starch, a major component in food waste, in NaCl(aq) solution under robust microwave heating, without adding catalysts and organic solvents to pursue green chemistry. The NaCl-microwave synergy was revealed via an innovative approach, by tracing and assimilating the data of microwave real-time power output, in-vessel pressure, and temperature gradient across the reactant matrix. The use of very diluted NaCl(aq) (0.05 wt%) increased the total product yields from 23.6 to 37.4 mol%, with tetra/tri/disaccharides and glucose being the major products after reaction at 200 °C for 15 min. The highest product yields of 65.9 mol% could be obtained in 1 wt% NaCl(aq). Fluctuation in the temperature measurement during ramping signified the NaCl-promoted gelatinisation, in which the reactant mixture exhibited rapid changes in viscosity. The 13C solid-state nuclear magnetic resonance spectroscopy suggested the possible selective interactions between NaCl and the free hydroxyl groups at C6 and C2,3,5 positions, whereas α(1 → 4) linkages were less assessable due to steric hindrance. The hydrolytic depolymerisation of starch was auto-catalysed by acid species generated in situ at the temperature holding stage. This step was accelerated by the presence of NaCl that might interact with and weaken the glycosidic bonds. From an energy perspective, the use of microwaves in place of conventional heating reduced the energy consumption by 70% to achieve the same products profile, as NaCl enabled superior dielectric heating via ionic loss. This study offers new insights into the NaCl-polysaccharide interactions and unique NaCl functions under microwave conditions, which are necessary fundamentals to develop green and energy-efficient biorefinery systems.


 Please wait while we load your content...
Please wait while we load your content...