Highly efficient light-driven methane coupling under ambient conditions based on an integrated design of a photocatalytic system†
Abstract
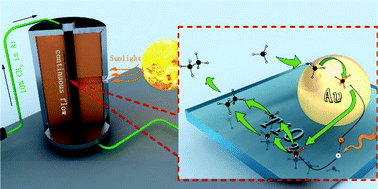
Direct non-oxidative coupling of methane (NOCM) is an effective way to produce hydrocarbons. However, this process usually requires a high temperature (≥1100 °C) to break the C–H bond of CH4 and suffers catalyst deactivation due to coke formation. Photocatalytic NOCM is an ideal strategy to solve these issues. Herein, we designed a novel photocatalytic methane coupling system consisting of a continuous flow reactor and metal-loaded TiO2 photocatalysts with light-diffuse-reflection-surfaces. It was found that Au/TiO2 was the best catalyst for the system due to the easy transport of photoelectrons from TiO2 to Au particles to inhibit the photoelectron–hole recombination. The yield of C2H6 reached 81.7 μmol gcatalyst−1 h−1 with higher than 95% selectivity over Au/TiO2 under simulated 1.5G sunlight irradiation and ambient conditions (room temperature and 1 atm), which is 174% larger than the highest reported value. Furthermore, DFT calculation results revealed that the methyl anion is a possible intermediate species for the formation of ethane.


 Please wait while we load your content...
Please wait while we load your content...