Calcium carbide as a dehydrating agent for the synthesis of carbamates, glycerol carbonate, and cyclic carbonates from carbon dioxide†
Abstract
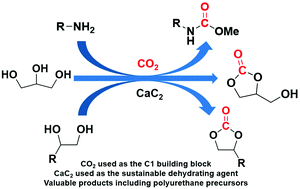
Carbon dioxide (CO2) is a nontoxic and inexpensive C1 building block, which can be used for the synthesis of valuable chemicals such as aromatic carbamates from anilines and methanol (MeOH), glycerol carbonate from glycerol, and cyclic carbonates from diols. However, these reactions generate water as the byproduct and suffer from thermodynamic limits, which lead to low yields. Calcium carbide (CaC2) is a renewable chemical, which can be recycled from calcium that is abundant in the Earth's crust. Furthermore, CaC2 rapidly reacts with water. In this work, we used CaC2 as a dehydrating agent for the direct synthesis of carbamates (including polyurethane precursors) from amines, CO2, and MeOH. All reagents were commercially available. In addition, CaC2 was employed for the synthesis of glycerol carbonate from glycerol and CO2 with a zinc catalyst and N-donor ligand. A similar protocol was applied to synthesize cyclic carbonates from diols and CO2.


 Please wait while we load your content...
Please wait while we load your content...