Nickel-catalyzed and Li-mediated regiospecific C–H arylation of benzothiophenes†
Abstract
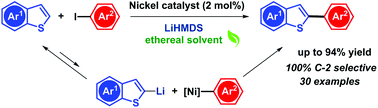
A nickel-based catalytic system for the regiospecific C2–H arylation of benzothiophene has been established. NiCl2(bpy) is used as a catalyst in combination with LiHMDS as a base in dioxane. The catalytic system is applicable to a variety of functionalized benzothiophenes, as well as other heteroarenes including thiophene, benzodithiophene, benzofuran and selenophene in combination with iodo aryl electrophiles. The role of LiHMDS as a uniquely potent base and a postulated mechanism are discussed. The applicability of this system is finally demonstrated for the synthesis of an intermediate of an active pharmaceutical ingredient.
- This article is part of the themed collection: Green Chemistry 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...