Microwave-assisted aqueous carbon–carbon cross-coupling reactions of aryl chlorides catalysed by reduced graphene oxide supported palladium nanoparticles†
Abstract
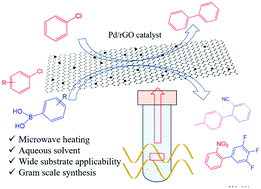
The use of low cost and readily available aryl chlorides as starting reactants in palladium-catalyzed carbon–carbon cross-coupling reactions has drawn significant research attention. However, previously reported heterogeneous palladium catalysts suffered from poor reactivity and harsh conditions. Also, valuable industrial products were rarely obtained in these catalytic systems to date. Herein, a simple and green in situ assembly and reduction approach was developed for the fabrication of reduced graphene oxide supported palladium nanoparticles (Pd/rGO). Owing to the abundant surface functional groups, Pd NPs were uniformly dispersed on the sheets of rGO with an average size of around 2.0 nm. Interestingly, under microwave irradiation, Pd/rGO can efficiently promote Ullmann and Suzuki coupling reactions by using aryl chlorides as the reactants in aqueous media, which showed even better catalytic performances than a homogeneous catalytic system. Notably, this mild reaction system can be demonstrated in the gram-scale synthesis of 4′-methyl-2-biphenylcarbonitrile and 2-nitro-3′,4′,5′-trifluoro-1,1′-biphenyl, which are important pharmaceutical intermediates of sartans and fluxapyroxad, respectively. Based on material characterization and control experiments, this remarkable catalytic performance could be ascribed to its robust microwave absorption ability, efficient electron transfer and unique two-dimensional structure. Furthermore, it was easily recycled and used repetitively at least six times without significant loss of its activity.


 Please wait while we load your content...
Please wait while we load your content...