Electrocatalytic three-component annulation-halosulfonylation of 1,6-enynes toward 1-indanones using sodium halides as both halogen sources and electrolytes†
Abstract
A new electrochemically induced three-component annulation-halosulfonylation of 1,6-enynes has been developed for stereoselective synthesis of 33 examples of 1-indanones with generally good yields under environmentally benign conditions. This electrochemical strategy can proceed in a simple undivided cell, avoiding any transition metal catalysts, chemical oxidants and additives. This reaction tolerates a wide scope of substrates, which provides a new and green access to fabricate important bioactive 1-indanone scaffolds. Notably, readily available and low-cost halogen salts play triple roles of an electrolyte and a redox catalyst as well as a halogenating reagent.
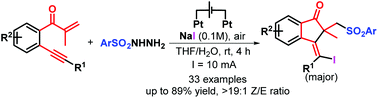


 Please wait while we load your content...
Please wait while we load your content...