Base-mediated benzannulation of α-cyanocrotonates with ynones: facile synthesis of benzonitriles and fluorenes†
Abstract
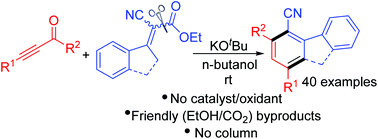
Here, we have demonstrated a strategic rapid approach for the synthesis of benzonitriles and cyanofluorenes via the [3 + 3] benzannulation of readily available alkynones and α-cyanocrotonates, a protocol par excellence for aryl nitriles. This decarboxylative annulation has been assisted solely by a base without the need for any catalyst. The only by-product is EtOH (and CO2) and the product is cleanly filtered from the contents after the reaction at an ambient temperature.


 Please wait while we load your content...
Please wait while we load your content...