Highly efficient oxidation of alcohols to carboxylic acids using a polyoxometalate-supported chromium(iii) catalyst and CO2†
Abstract
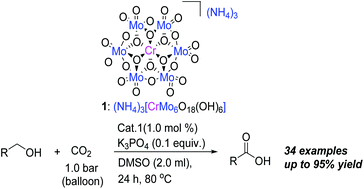
Direct catalytic oxidation of alcohols to carboxylic acids is very attractive, but economical catalysis systems have not yet been well established. Here, we show that a pure inorganic ligand-supported chromium compound, (NH4)3[CrMo6O18(OH)6] (simplified as CrMo6), could be used to effectively promote this type of reaction in the presence of CO2. In almost all cases, oxidation of various alcohols (aromatic and aliphatic) could be achieved under mild conditions, and the corresponding carboxylic acids can be achieved in high yield. The chromium catalyst 1 can be reused several times with little loss of activity. Mechanism study and control reactions demonstrate that the acidification proceeds via the key oxidative immediate of aldehydes.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...