An electrochemical oxidative multicomponent cascade annulation of ketones and amines used to produce imidazoles†
Abstract
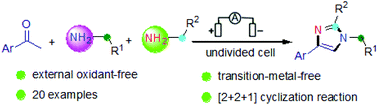
An electrochemical dehydrogenative [2 + 2 + 1] annulation used for the synthesis of imidazoles has been developed under undivided electrolytic conditions. In an undivided cell, aryl ketones and amines can smoothly participate in this transformation to furnish a variety of substituted imidazoles. The reaction avoids the use of both transition-metal catalysts and peroxide reagents, which makes it more sustainable and renewable.


 Please wait while we load your content...
Please wait while we load your content...