Mechanochemical cleavage of lignin models and lignin via oxidation and a subsequent base-catalyzed strategy†
Abstract
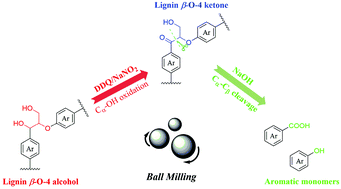
Mechanochemical cleavage of lignin dimer model compounds to phenolic monomers has been developed via a two-step strategy under milling conditions. In the first step of this process, the secondary benzylic alcohol of lignin β-O-4 linkages was selectively oxidized to the corresponding ketones over a 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)/NaNO2 catalytic system under milling conditions. In the subsequent step, mechanochemical selective cleavage of the Cβ–O bonds and Cα–Cβ bonds of lignin β-O-4 ketones to acids and phenols was promoted by NaOH-catalyzed depolymerization. In addition, this two-step strategy was performed to depolymerize organosolv birch lignin, giving aromatic monomers with good selectivity for syringate. This approach provides an efficient method to convert the β-O-4 linkages of lignin to valuable aromatic monomers under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...