Scalable synthesis and polymerisation of a β-angelica lactone derived monomer†
Abstract
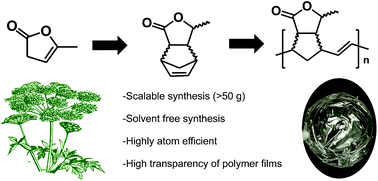
Bio-based levulinic acid is easily ring-closed to α-angelica lactone (α-AL). α-AL can be isomerized to the conjugated β-AL under the influence of base, but since this is an equilibrium mixture it is very hard to devise a scalable process that would give pure β-AL. This problem was circumvented by distilling the equilibrium mixture to obtain a 90 : 10 mixture of β- and α-AL in 88% yield. This mixture was used for Diels–Alder reactions on 3 terpenes and on cyclopentadiene in up to 100 g scale. The latter DA adduct was subjected to a ROMP reaction catalysed by the Grubbs II catalyst. The resulting polymer has some similarities to poly-norbornene but is more polar. The polymer can be processed into films with very good transparency.


 Please wait while we load your content...
Please wait while we load your content...