Alkene versus alkyne reactivity in unactivated 1,6-enynes: regio- and chemoselective radical cyclization with chalcogens under metal- and oxidant-free conditions†
Abstract
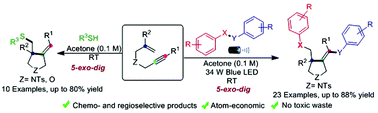
Herein, we have developed metal and oxidant-free visible light-promoted alkene vs. alkyne regio- and chemoselective radical cascade cyclization of electronically unbiased 1,6-enynes with chalcogens to synthesize substituted pyrrolidines bearing chalcogens. The reaction generated three new bonds, namely, C–SO2, C–C, and C–Se under extremely mild conditions. Furthermore, we achieved regio- and chemoselective mono-addition of aromatic thiophenols with unactivated 1,6-enynes. The key features of this protocol are broad substrate scope, environment-friendly conditions, operational simplicity, atom economy, and amenability to gram-scale synthesis. The mechanistic studies corroborate that the reaction proceeds via a radical pathway.


 Please wait while we load your content...
Please wait while we load your content...