Radical cyclization of 1,6-dienes with azobis(alkylcarbonitriles) on water under additive-free conditions†
Abstract
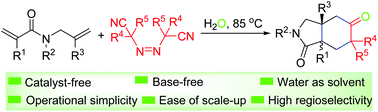
Without using any additives, a practical and eco-friendly methodology has been realized for the tandem double cyclization of 1,6-dienes with easily accessible azobis(alkylcarbonitriles) on water. This chemistry has mild reaction conditions, employing water as the sole solvent, with high regioselectivity and ease of scale-up. Moreover, this is the first example of 1,n-diene cyclization using azobis(alkylcarbonitriles) as a two-carbon unit for the construction of polycyclic skeletons.


 Please wait while we load your content...
Please wait while we load your content...