Transition metal- and solvent-free double hydroboration of nitriles†
Abstract
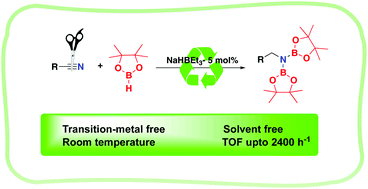
A highly efficient, room temperature double hydroboration of nitriles under transition metal-free and solvent-free conditions is reported. Sodium triethylborohydride is used as a catalyst and yields up to 99% are disclosed. Mechanistic studies reveal the reaction proceeds in a stepwise manner with initial formation of a boryl-imine which undergoes a second hydroboration to afford a diborylated amine product.


 Please wait while we load your content...
Please wait while we load your content...