Photocatalyst- and transition-metal-free α-allylation of N-aryl tetrahydroisoquinolines mediated by visible light†
Abstract
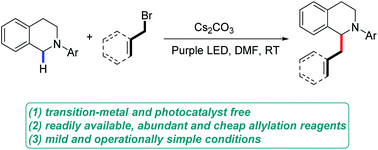
A convenient and efficient α-allylation of N-aryl tetrahydroisoquinolines has been achieved. This transformation can be realized under only visible light irradiation without the aid of transition metals or photocatalysts. The mechanism involves a novel in situ-generated electron-donor–acceptor (EDA) complex between the N-aryl tetrahydroisoquinolines and an allyl or a benzyl bromide. Irradiation with purple light triggered single-electron transfer (SET) from the N-aryl tetrahydroisoquinolines to the allyl or benzyl bromide of the EDA complex, inducing the formation of the corresponding allyl or benzyl radical and the subsequent radical–radical coupling. This approach represents the first example of a photocatalyst- and transition-metal-free α-allylic and benzylic functionalization of N-aryl tetrahydroisoquinolines.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...