Tailor-made β-glucosidase with increased activity at lower temperature without loss of stability and glucose tolerance†
Abstract
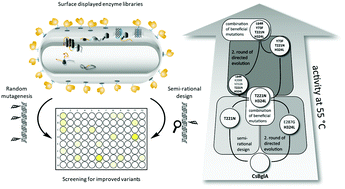
The hydrolysis of cellobiose by β-glucosidases represents the crucial step in the degradation of lignocellulosic biomass and is therefore a major target for improvement by enzyme engineering. BglA from Caldicellulosiruptor saccharolyticus (CsBglA) is among the β-glucosidases with highest known activity and thermostability, however with a temperature optimum of 70–80 °C. To improve the enzyme's activity at moderate temperatures, a semi-rational approach based on homology modeling, conservation scoring and subsequent site-saturation mutagenesis was combined with random mutagenesis, resulting in the variant CsBglA-LYTH with 150% improvement of kcat/KM at 55 °C, a specific activity of 504 U mg−1 (CsBglA: 359 U mg−1) and a KM-value of 37.1 mM (CsBglA: 61.2 mM). The temperature optimum of the enzyme was altered from 80 °C to 65 °C while retaining stability at the temperature of 55 °C as applied for cellulose hydrolysis. To our knowledge, this makes CsBglA-LYTH the bacterial β-glucosidase with highest activity and stability.


 Please wait while we load your content...
Please wait while we load your content...