Temperature-controlled regioselective thiolation of 2-indolylmethanols under aqueous micellar conditions†
Abstract
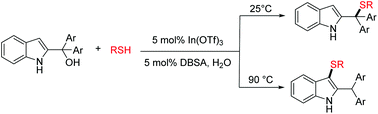
A sustainable and regioselective synthesis of highly functionalized indole-based sulfides from thiols and 2-indolymethanols is described. Notably, this reaction can be performed in the presence of 5 mol% In(OTf)3 under aqueous micellar conditions using an inexpensive surfactant DBSA. This protocol offers several advantages including excellent regioselectivities, being organic solvent-free, ease of scale-up, broad substrate scope, and recycling of the aqueous reaction medium and catalyst.


 Please wait while we load your content...
Please wait while we load your content...