Regio/site-selective alkylation of substrates containing a cis-, 1,2- or 1,3-diol with ferric chloride and dipivaloylmethane as the catalytic system†
Abstract
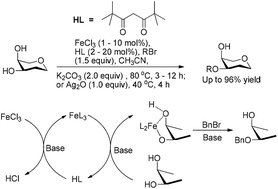
In this study, we reported the regio/site-selective alkylation of substrates containing a cis-, 1,2- or 1,3-diol with FeCl3 as a key catalyst. A catalytic system consisting of FeCl3 (0.01–0.1 equiv.) and dipivaloylmethane (FeCl3/dipivaloylmethane = 1/2) was used to catalyze the alkylation in the presence of a base. The produced selectivities and isolated yields were similar to those obtained by methods using the same amount of FeL3 (L = acylacetone ligand) as the catalyst in most cases. The previously reported FeL3 catalysts for alkylation are not commercially available and have to be synthesized prior to use. In contrast, FeCl3 and dipivaloylmethane (Hdipm) are very common and inexpensive nontoxic reagents in the lab, thereby making the method much greener and easier to handle. Mechanism studies confirmed for the first time that FeCl3 initially reacts with two equivalents of Hdipm to form [Fe(dipm)3] in the presence of a base in acetonitrile, followed by the formation of a five or six-membered ring intermediate between [Fe(dipm)3] and two hydroxyl groups of the substrate. A subsequent reaction between the cyclic intermediate and the alkylating agent results in selective alkylation of the substrate.


 Please wait while we load your content...
Please wait while we load your content...