Tandem selective reduction of nitroarenes catalyzed by palladium nanoclusters†
Abstract
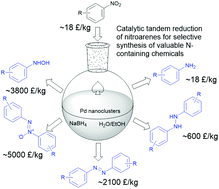
We report a catalytic tandem reduction of nitroarenes by sodium borohydride (NaBH4) in aqueous solution under ambient conditions, which can selectively produce five categories of nitrogen-containing compounds: anilines, N-aryl hydroxylamines, azoxy-, azo- and hydrazo-compounds. The catalyst is in situ-generated ultrasmall palladium nanoclusters (Pd NCs, diameter of 1.3 ± 0.3 nm) from the reduction of Pd(OAc)2 by NaBH4. These highly active Pd NCs are stabilized by surface-coordinated nitroarenes, which inhibit the further growth and aggregation of Pd NCs. By controlling the concentration of Pd(OAc)2 (0.1–0.5 mol% of nitroarene) and NaBH4, the water/ethanol solvent ratio and the tandem reaction sequence, each of the five categories of N-containing compounds can be obtained with excellent yields (up to 98%) in less than 30 min at room temperature. This tunable catalytic tandem reaction works efficiently with a broad range of nitroarene substrates and offers a green and sustainable method for the rapid and large-scale production of valuable N-containing chemicals.


 Please wait while we load your content...
Please wait while we load your content...