Catalyst-free, direct electrochemical synthesis of annulated medium-sized lactams through C–C bond cleavage†
Abstract
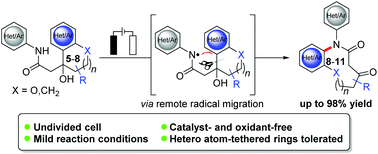
A catalyst-free, direct electrochemical synthesis of synthetically challenging medium-sized lactams through C–C bond cleavage has been developed. In contrast to previous typical amidyl radical cyclization, this electrosynthetic approach enabled step-economical ring expansion through a unique remote amidyl migration under mild, metal- and external-oxidant-free conditions in a simple undivided cell. The strategy features unparalleled broad substrate scope with all ring sizes of (hetero)aryl-fused 8–11-membered rings and hetero atom-tethered rings, high yields, and good functional group tolerance. Our experimental and computational findings provided strong support for a SET-based reaction manifold.


 Please wait while we load your content...
Please wait while we load your content...