Synthesis of α-enaminones from cyclic ketones and anilines using oxoammonium salt as an oxygen transfer reagent†
Abstract
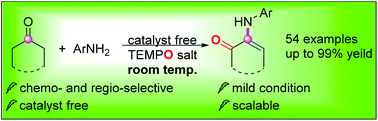
A convenient and straightforward transformation of cyclic ketones with anilines at room temperature has been developed using oxoammonium salt TEMPO+PF6− as an oxidant. This method enabled the synthesis of a broad range of α-enaminones. The 18O-labeling experiment demonstrated that oxoammonium salt served as the oxygen transfer reagent.


 Please wait while we load your content...
Please wait while we load your content...