Electroactivated alkylation of amines with alcohols via both direct and indirect borrowing hydrogen mechanisms†
Abstract
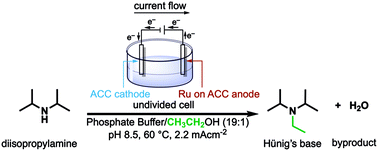
A green, efficient N-alkylation of amines with simple alcohols has been achieved in aqueous solution via an electrochemical version of the so-called “borrowing hydrogen methodology”. Catalyzed by Ru on activated carbon cloth (Ru/ACC), the reaction works well with methanol, and with primary and secondary alcohols. Alkylation can be accomplished by either of two different electrocatalytic processes: (1) in an undivided cell, alcohol (present in excess) is oxidized at the Ru/ACC anode; the aldehyde or ketone product condenses with the amine; and the resulting imine is reduced at an ACC cathode, combining with protons released by the oxidation. This process consumes stoichiometric quantities of current. (2) In a membrane-divided cell, the current-activated Ru/ACC cathode effects direct C–H activation of the alcohol; the resulting carbonyl species, either free or still surface-adsorbed, condenses with amine to form imine and is reduced as in (1). These alcohol activation processes can alkylate primary and secondary aliphatic amines, as well as ammonia itself at 25–70 °C and ambient pressure.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...
