An unconventional sulfur-to-selenium-to-carbon radical transfer: chemo-and regioselective cyclization of yne-ynamides†
Abstract
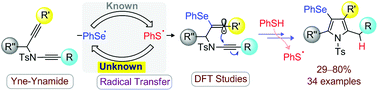
An uncommon sulfur → selenium → carbon radical transfer process is employed to develop an unprecedented selenyl radical-mediated regioselective cyclization of yne-tethered-ynamides. Density functional theory studies and HRMS experiments are used to establish a reactivity scale between thiyl and selenyl radicals. The unique features of this transformation include, (1) the chemoselective reactivity of RSe˙ over RS˙, (2) regioselective RSe˙ attack on alkyne over ynamide, (3) 5-exo-dig cyclization of yne-ynamide to unusual 4-selenyl-pyrroles, and (4) the green synthetic method. The reaction of methyldiselenide with yne-ynamides to methylselenopyrroles is also described.


 Please wait while we load your content...
Please wait while we load your content...