A palladium-catalyzed oxidative aminocarbonylation reaction of alkynone O-methyloximes with amines and CO in PEG-400†
Abstract
A palladium-catalyzed oxidative aminocarbonylation of O-methyloximes and amines under aerobic conditions with PEG-400 as an environmentally benign medium was accomplished. This novel protocol provides the first straightforward synthetic method for the assembly of structurally diverse 4-carboxamide isoxazoles with high atom- and step-economy and exceptional functional group tolerance. Notably, the employment of ionic liquids as an additive, with air as the sole oxidant, without ligands is an attractive feature of the present approach.
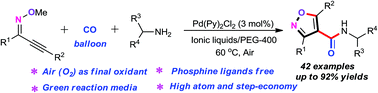


 Please wait while we load your content...
Please wait while we load your content...