Continuous-flow photo-induced decarboxylative annulative access to fused imidazole derivatives via a microreactor containing immobilized ruthenium†
Abstract
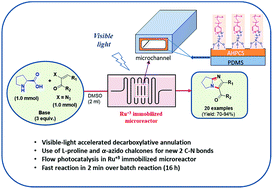
Visible-light-driven continuous-flow decarboxylative annulation was achieved and used along with a microreactor containing immobilized ruthenium catalyst to construct valuable fused imidazole derivatives with high yields under an open atmosphere. Notably, this chemistry included the use of L-proline and α-azidochalcone as precursors of an α-amino radical and 2H-azirine via photo-induced decarboxylation and denitrogenation, respectively, to give the annulated imidazole derivatives as a result of the formation of two new C–N bonds. Moreover the novel, environmentally benign and efficient continuous-flow protocol was further improved by carrying out the reaction in a polydimethylsiloxane (PDMS) microreactor with immobilized Ru3+ under fluorescent or white LED light, enabling excellent yields (70–94%) at a reaction time (2 min) significantly shorter than that (16 h) of the batch protocol.


 Please wait while we load your content...
Please wait while we load your content...