In water alkylation of amines with alcohols through a borrowing hydrogen process catalysed by ruthenium nanoparticles†
Abstract
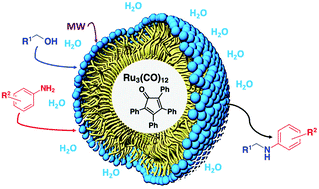
A simple and environmentally benign procedure for the synthesis of secondary amines in water has been developed. Combining Ru3(CO)12, tetraphenylcyclopentadienone and a small quantity of TGPS-750-M surfactant, primary and secondary alcohols were alkylated at N employing equimolar amounts of aromatic amines in water. The reaction occurs under microwave (MW) dielectric heating with high conversion and high yield. When required, the use of biomass-derived 2-MeTHF or GVL as a co-solvent is possible. Under the influence of MWs, a Ru nanoparticle–nanomicelle combination was formed acting as an effective and recyclable catalyst. This protocol was also employed for “in water” cyclisation to synthesise biologically relevant pyrrolobenzodiazepines (PBDs).


 Please wait while we load your content...
Please wait while we load your content...