Photochemical oxidation of benzylic primary and secondary alcohols utilizing air as the oxidant†
Abstract
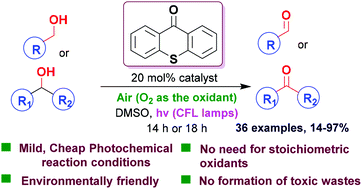
A mild and green photochemical protocol for the oxidation of alcohols to aldehydes and ketones was developed. Utilizing thioxanthenone as the photocatalyst, molecular oxygen from air as the oxidant and cheap household lamps or sunlight as the light source, a variety of primary and secondary alcohols were converted into the corresponding aldehydes or ketones in low to excellent yields. The reaction mechanism was extensively studied.


 Please wait while we load your content...
Please wait while we load your content...