Solvometallurgical process for extraction of copper from chalcopyrite and other sulfidic ore minerals†
Abstract
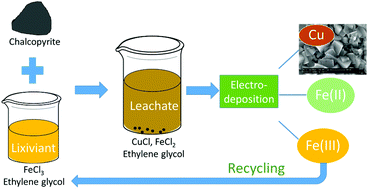
Extraction of copper from sufidic ores, either by pyrometallurgy or hydrometallurgy, has various limitations. In this study, a solvometallurgical process for the extraction of copper from sulfidic ore minerals (chalcopyrite, bornite, chalcocite and digenite) was developed by using an organic lixiviant (FeCl3 as oxidizing agent and ethylene glycol (EG) as organic solvent). All the studied copper sulfide minerals could be leached efficiently with a FeCl3–EG solution. Other lixiviant systems, namely CuCl2–EG, FeCl3–ethanol and FeCl3–propylene glycol could also extract copper, but they did not perform as well as the FeCl3–EG solutions. The mechanistic study of chalcopyrite leaching in FeCl3–EG solutions confirmed that the leaching products of chalcopyrite were FeCl2, CuCl and solid elemental sulfur, where the Fe(II) and Cu(I) were quantified by UV-Vis absorption spectroscopy and solid sulfur was identified by powder XRD. A kinetic study showed that the leaching process was a surface chemical controlled process and the apparent activation energy was calculated to be 60.1 kJ mol−1. Subsequently, electrodeposition of copper from the pregnant leachate was investigated, and SEM-EDX analysis showed that uniform cubic crystalline deposits of pure copper were produced. Meanwhile, the Fe(III) was regenerated by oxidizing Fe(II) at the anode, with a Morgane membrane in between two electrode compartments to prevent the transfer of Fe(III) to the cathode. Finally, a closed-loop solvometallurgical process was designed with three operational steps: leaching, electrodeposition and removal of Fe(II). The regeneration of the FeCl3–EG solution and the use of EG contribute to the sustainability and the greenness of the process.


 Please wait while we load your content...
Please wait while we load your content...