Conformational changes in bovine α-lactalbumin and β-lactoglobulin evoked by interaction with C18 unsaturated fatty acids provide insights into increased allergic potential†
Abstract
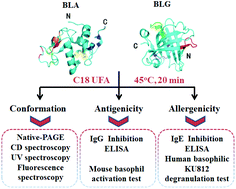
Bovine α-lactalbumin (BLA) and β-lactoglobulin (BLG) are the most common and severe food allergens in milk and they can bind C18 unsaturated fatty acids (UFAs) and their bioactivities were changed. This study aims to determine the effects of C18 UFAs on the structures of BLA and BLG and their allergic properties, such as antigenicity and allergenicity. We reveal that C18 UFAs can efficiently promote the gradual unfolding of the structures of BLA and BLG and increase their hydrophobicity. Moreover, the IgG binding ability and the expression of IgG-dependent activation marker CD200R3 on basophils were remarkably promoted after C18 UFA treatment. Finally, we also observed that C18 UFAs can enhance the IgE binding ability and the degranulation capacity of human basophil KU812 cells (intracellular Ca2+, histamine, β-Hex, and IL-6). Collectively, these results suggested that C18 UFAs changed the structures of BLA and BLG, which contributed to their increased allergic potential.


 Please wait while we load your content...
Please wait while we load your content...