Number of galloyl moieties and molecular flexibility are both important in alpha-amylase inhibition by galloyl-based polyphenols
Abstract
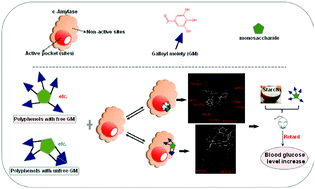
The inhibition of porcine pancreatic α-amylase (PPA) by 9 galloyl-based polyphenols was evaluated via initial digestion velocity, IC50, inhibition kinetics, fluorescence quenching and molecular docking studies. The results show that the polyphenols with free galloyl moieties (GMs) exhibited a much higher inhibition effect than that with bound GMs. The inhibition was competitive mode and increased with an increase in the number of free GMs. Interestingly, all the constants that indicate the binding affinity of polyphenols to PPA, including the reciprocal of the competitive inhibition constant (1/Kic), fluorescence quenching constant (KFQ) and binding energy (Eb), increased with an increase in the number of free GMs. Besides, the respective order of 1/Kic, KFQ and Eb for the galloyl-based polyphenols was contrary to that of IC50, indicating that the polyphenol–PPA binding interactions resulted in enzyme inhibition. In addition, the binding interactions were suggested to result from the hydrogen bonding and π–π conjugation forces between the free GMs and amino acid residues at the active sites of PPA, whereas the chemical linkage of the GMs with additional polyphenol groups limited these interactions. Furthermore, the hypoglycemic effects of two polyphenols with 5 free GMs indicate that GMs may be considered a functional fragment involved in the alleviation of the symptoms of type II diabetes through α-amylase inhibition.
- This article is part of the themed collection: Food & Function Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...