Influence of sodium pyrophosphate on the physicochemical and gelling properties of myofibrillar proteins under hydroxyl radical-induced oxidative stress
Abstract
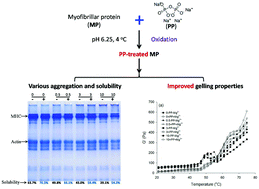
Porcine myofibrillar proteins (MP) with or without sodium pyrophosphate (PP) were oxidatively stressed in hydroxyl radical (˙OH)-generating systems (10 μM FeCl3, 100 μM ascorbic acid, and 0, 0.5, 3, 10 mM H2O2) at 4 °C for 12 h. The results showed significant protein oxidation under the ˙OH stress, indicated by the modification of amino acid side chain groups and the aggregation of MP, which led to losses in gelling properties of MP especially at high dosages of H2O2 (3–10 mM). The PP addition effectively suppressed ˙OH induced lipid oxidation (as evidenced by TBARS values) in MP, but the inhibitory effect on protein oxidation was limited. In fact, the PP treatment with a high level of H2O2 (10 mM) tended to promote protein unfolding and aggregation in the tested systems. However, a significantly (P < 0.05) improved protein solubility was found in all tested systems with added PP. The PP treated MP gels exhibited a more compact and orderly microstructure, which may explain the reduced cooking loss and improved gel strength.


 Please wait while we load your content...
Please wait while we load your content...