Controllable membrane remodeling by a modified fragment of the apoptotic protein Bax
Abstract
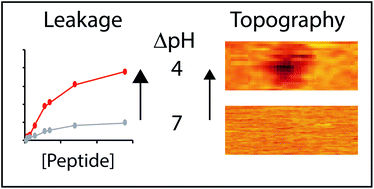
Intrinsic apoptosis is orchestrated by a group of proteins that mediate the coordinated disruption of mitochondrial membranes. Bax is a multi-domain protein that, upon activation, disrupts the integrity of the mitochondrial outer membrane by forming pores. We strategically introduced glutamic acids into a short sequence of the Bax protein that constitutively creates membrane pores. The resulting BaxE5 peptide efficiently permeabilizes membranes at acidic pH, showing low permeabilization at neutral pH. Atomic force microscopy (AFM) imaging showed that at acidic pH BaxE5 established several membrane remodeling modalities that progressively disturbed the integrity of the lipid bilayer. The AFM data offers vistas on the membrane disruption process, which starts with pore formation and progresses through localized exposure of membrane monolayers leading to stable and small (height ∼ 16 Å) lipid–peptide complexes. The different types of membrane morphology observed in the presence of BaxE5 suggest that the peptide can establish different types of membrane interactions. BaxE5 adopts a rare unstructured conformation when bound to membranes, which might facilitate the dynamic transition between those different states, and then promote membrane digestion.
- This article is part of the themed collection: Peptide-membrane interactions


 Please wait while we load your content...
Please wait while we load your content...