Cooperative phenomenon of vapochromism and proton conduction of luminescent Pt(ii) complexes for the visualisation of proton conductivity†
Abstract
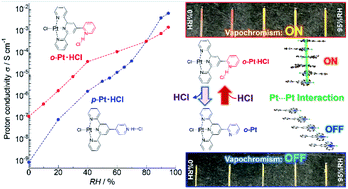
The luminescent and proton conductive Pt(II) complex [PtCl(tpy-o-py)]Cl and its HCl adduct [PtCl(tpy-o-pyH)]Cl2 (o-Pt and o-Pt·HCl, respectively; tpy-o-py = 2,2′:6′,2′′-terpyridine-6′,2′′′-pyridine) were synthesised and their crystal structures, vapochromic behaviour, and proton conduction, were investigated and compared to those of the para isomers [PtCl(tpy-p-py)]Cl and [PtCl(tpy-p-pyH)]Cl2 (p-Pt and p-Pt·HCl, respectively; tpy-p-py = 2,2′:6′,2′′-terpyridine-4′,4′′′-pyridine). X-ray structure analysis revealed that the intermolecular metallophilic (Pt⋯Pt) interaction was negligible in o-Pt but effective in o-Pt·HCl. Reversible transformation between o-Pt and o-Pt·HCl coupled with significant colour and luminescence changes was achieved by four different external stimuli, namely: exposure of o-Pt to humid HCl gas to form o-Pt·HCl, heating, exposure to MeOH vapour, and finally drying in air to regenerate the original o-Pt. The intraligand π–π* orange emission observed for o-Pt exhibited negligible dependence on the relative humidity (RH). Conversely, o-Pt·HCl exhibited red metal–metal-to-ligand charge-transfer (MMLCT) phosphorescence at 725 nm, originating from effective intermolecular Pt–Pt interactions, and interesting vapochromic behaviour that was dependent on the RH. Notably, o-Pt·HCl presented higher conductivity than the p-Pt·HCl isomer at RH < 80%. This trend was reversed at RH values > 80%, probably owing to the second water-adsorption-induced transformation of p-Pt·HCl. The cooperative phenomenon between the proton conduction and vapochromic behaviour observed for both o-Pt·HCl and p-Pt·HCl should allow the visualisation of the proton-conducting pathway, without the need for a bulk electrode, via the absorption and emission colours at both macroscopic and microscopic levels.
- This article is part of the themed collection: Cooperative phenomena in framework materials


 Please wait while we load your content...
Please wait while we load your content...