The effect of H2 : N2 ratio on the NH3 synthesis rate and on process economics over the Co3Mo3N catalyst
Abstract
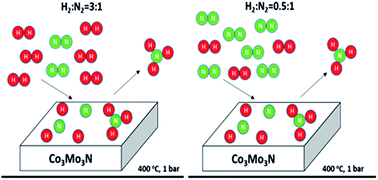
In this study, the process economics of ammonia synthesis over Co3Mo3N was investigated by searching for an optimum feed stoichiometry. From ammonia synthesis rate measurements at atmospheric pressure and 400 °C over Co3Mo3N, it was found that the rate was independent of H2 : N2 stoichiometry for stoichiometries above 0.5 : 1. For H2 : N2 stoichiometries below 0.5 : 1, there was a linear dependency of ammonia synthesis rate on the H2 : N2 stoichiometry. Static measurements of hydrogen adsorption isotherms at 25, 50, and 100 °C revealed that the adsorbed amounts of strongly bound hydrogen over the Co3Mo3N surface were saturated at around 100 Torr hydrogen pressure. This pressure corresponds to the partial pressure of hydrogen when the H2 : N2 stoichiometry is around 0.5 : 1, confirming the role of strongly bound hydrogen in ammonia synthesis. These results were used to modify an existing kinetic expression to be used in a conceptual design, based on a late mixing strategy for the hydrogen stream. This conceptual design and its economic analysis revealed that using low hydrogen stoichiometries can cut the investment and operating costs by a factor of 2.
- This article is part of the themed collection: Reaction mechanisms in catalysis


 Please wait while we load your content...
Please wait while we load your content...