Quantum proton tunneling in multi-electron/-proton transfer electrode processes
Abstract
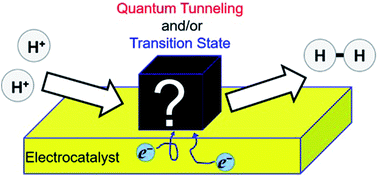
Quantum proton tunneling (QPT) in two representative multi-electron/-proton transfer electrode processes, i.e. hydrogen evolution reaction (HER) and oxygen reduction reaction (ORR), was investigated using polycrystalline platinum (pcPt) and gold (pcAu) electrodes at 298 kelvin (K). To observe quantum effects in the electrode processes, the hydrogen/deuterium kinetic isotope effect constant ratio (≡KH/D) was measured in various conditions. For the HER in both acidic and alkaline conditions, results show that the pcPt exhibits a negligible or weak QPT evident by the small value of KH/D (1 < KH/D < 3), which indicates that the semiclassical transition state theory (SC-TST) scheme dominates the rate-determining step (RDS). For pcAu in an alkaline condition, the KH/D was a small value of ca. 1 at a low η region around 0.2 V. However, at a high η region >0.6 V, a high KH/D (>13) was obtained. These results suggest a transition of the electrode process from SC-TST to a full QTP in the RDS on increasing the overpotential. For ORR with pcPt, KH/D higher than the theoretical maximum in SC-TST was observed in the alkaline condition at a low overpotential region. A primitive but robust theoretical analysis suggests that the QPT governs the rate-determining step of ORR in this condition. However, this full QPT path transits to the classical in a higher overpotential region. Therefore, contrary to the HER on pcAu in alkaline, the electrode process shows a transition from a full QPT to SC-TST on increasing the overpotential. No QPT in ORR on a pcPt electrode was observed in an acidic condition. This report describes that the QPT in surface electrochemical systems is strongly affected by the choice of system. Although several systems show a clear manifestation of QPT in the electrode processes and also primitive interpretations can be made of these observations, deriving a fine molecular-level picture of the results including several complicated effects remains challenging. However, the observations suggest that selection of a full QPT path might be affected strongly by different microscopic proton transfer mechanisms, i.e. proton transfer from hydronium ion or water molecules.
- This article is part of the themed collection: Quantum effects in complex systems


 Please wait while we load your content...
Please wait while we load your content...