Chlorination of N,N-dimethylhydrazine compounds: reaction kinetics, mechanisms, and implications for controlling N-nitrosodimethylamine formation during ozonation†
Abstract
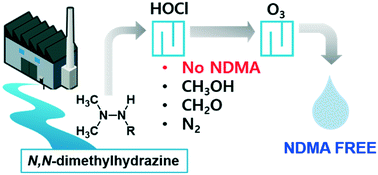
N,N-Dimethylhydrazine ((CH3)2–N–N–) compounds in water produce toxic N-nitrosodimethylamine (NDMA) during ozonation, thus need removing from water before ozonation. Chlorination can degrade N,N-dimethylhydrazines to give products that do not form NDMA during ozonation, but the chemistry is poorly understood. We studied the kinetics of and mechanisms involved in reactions between free available chlorine (FAC) and unsymmetrical dimethylhydrazine (UDMH) and daminozide (DMZ) (N,N-dimethylhydrazine compounds) and the efficiency with which FAC decreased the ozone–NDMA-formation potentials of UDMH and DMZ. FAC reacted quickly with UDMH (k = 4 × 105 M−1 s−1) and moderately with DMZ (k = 66 M−1 s−1) at pH 7. Formaldehyde and methanol were the main UDMH and DMZ degradation products, and succinic acid was also an important DMZ degradation product. Negligible NDMA formed (<0.01%) through FAC reacting with UDMH and DMZ. Ozonation of the chlorinated UDMH and DMZ samples gave much less (<7%) NDMA formation compared to the parent UDMH or DMZ. The possible reaction mechanisms involve a N–Cl adduct forming through FAC attacking a nitrogen atom on the primary amine of UDMH or the amide of DMZ, which decomposes via intermediate N,N-dimethyldiazene to give mono-methylhydrazine and formaldehyde. FAC then reacts with both nitrogen atoms in mono-methylhydrazine, which decomposes via intermediate diazomethane to give methanol and molecular nitrogen. FAC doses of 3–5 mg Cl2 per L fully eliminated DMZ and its ozone–NDMA-formation potential from river water and wastewater effluents, but ammonia or dissolved organic matter decreased the elimination efficiency of the ozone–NDMA-formation potential. Overall, chlorination was a feasible option to eliminate N,N-dimethylhydrazine-type ozone–NDMA precursors.
- This article is part of the themed collection: Drinking water oxidation and disinfection processes


 Please wait while we load your content...
Please wait while we load your content...