Advanced electrochemical oxidation for the simultaneous removal of manganese and generation of permanganate oxidant†
Abstract
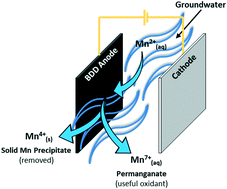
Emerging electrochemical systems, such as advanced electro-oxidation, provide a potentially powerful alternative to conventional oxidation processes which can often be unsuitable for small, remote and decentralised system applications. The one electro-oxidation process, which may be well suited for these applications, is the use of high oxygen overpotential boron-doped diamond (BDD) electrodes, as a pre-oxidation step for the removal of various raw water contaminants. While BDD electro-oxidation has been studied extensively for the abatement of organic micropollutants, its application as a pre-oxidation technology for the removal of soluble manganese (Mn2+) in source waters for drinking water supply, has not been reported to-date. In this study, we summarise the results of tests using a bench-scale electro-oxidation system and synthetic Mn2+ solutions in order to consider the simultaneous removal of manganese and the generation of permanganate. The results showed that total manganese was reduced by 9.1, 38.7 and 57.4% at current densities of 10, 40 and 80 mA cm−2, respectively, with an initial Mn2+ concentration of 39 μM. Increased Mn removal at higher current density was attributed to increased generation of, and reaction with, hydroxyl radicals, indicated by a direct proportional relationship between pseudo-first order reaction rate constants for methanol (˙OH radical scavenger) and current density. A mathematical model was developed to describe Mn removal under mass transport limitations, and was found to correlate well with experimental data. Finally, a completely novel synthesis pathway for the generation of permanganate species (Mn7+) is presented, whereby concentrations up to 0.9 μM were synthesised from Mn2+ in circumneutral conditions.
- This article is part of the themed collection: Drinking water oxidation and disinfection processes


 Please wait while we load your content...
Please wait while we load your content...