Electrochemical simultaneous denitrification and removal of phosphorus from the effluent of a municipal wastewater treatment plant using cheap metal electrodes†
Abstract
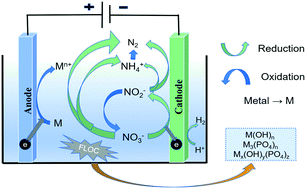
Nitrate and phosphate in the effluent of a municipal wastewater treatment plant (MWTP) were electrochemically removed with cheap metal (aluminum and iron) electrodes. The effects of electric current density, electrode spacing, electrolyte, electrolysis time and electrode material on nitrate and phosphate removal were investigated. The results demonstrated the feasibility of simultaneous removal of nitrate and phosphate by electroreduction and electrocoagulation using an aluminum or iron electrode. The optimum reaction conditions were determined as follows: the electric current density was 8 mA cm−2 and the electrolysis time was 1.5 h. The best removal performance of nitrate and phosphate could be obtained by using an aluminum sheet as a cathode and an anode. Under the above conditions, the removal rate of nitrate and phosphate was 100%, and 85% nitrate was converted to nitrogen gas. The XRD, FTIR and FESEM characterization spectra of the precipitate produced in the electrolytic cell were analyzed, and it was found that the main removal mechanisms of phosphate were the co-precipitation of metal ions, phosphate ions and hydroxide ions in solution and the adsorption of phosphate by hydroxyl metal complexes. Compared with an iron sheet, an aluminum sheet is less consumed in the electrolysis process with the same treatment effect. The electrolysis of actual wastewater was studied to verify the experimental conclusions. This study showed that the total nitrogen concentration in wastewater could be reduced to below 15 mg L−1 by aluminum electrolysis for 1.5 h at 8 mA cm−2. And the ammonia concentration was below 5 mg L−1. In other words, this study provided an economical and efficient alternative method for simultaneous removal of nitrate and phosphorus from the effluent of MWTPs.
- This article is part of the themed collection: SDG6: Clean water & sanitation


 Please wait while we load your content...
Please wait while we load your content...