Reactions of pyrrole, imidazole, and pyrazole with ozone: kinetics and mechanisms†
Abstract
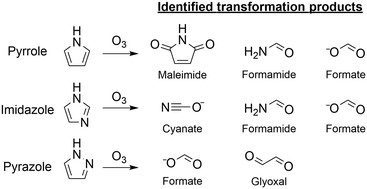
Five-membered nitrogen-containing heterocyclic compounds (azoles) belong to potential moieties in complex structures where transformations during ozonation can occur. This study focused on the azole–ozone chemistry of pyrrole, imidazole, and pyrazole as model compounds. Reaction kinetics and ozonation products were determined by kinetic and analytical methods including NMR, LC-HRMS/MS, HPLC-UV, and IC-MS. Analyses of reactive oxygen species (1O2, ˙OH, H2O2), quantum chemical computations (Gibbs energies), and kinetic simulations were used to further support the proposed reaction mechanisms. The species-specific second-order rate constants for the reactions of ozone with pyrrole and imidazole were (1.4 ± 1.1) × 106 M−1 s−1 and (2.3 ± 0.1) × 105 M−1 s−1, respectively. Pyrazole reacted more slowly with ozone at pH 7 (kapp = (5.6 ± 0.9) × 101 M−1 s−1). Maleimide was an identified product of pyrrole with a 34% yield. Together with other products, formate, formamide, and glyoxal, C and N mass balances of ∼50% were achieved. Imidazole reacted with ozone to cyanate, formamide, and formate (∼100% yields per transformed imidazole, respectively) with a closed mass balance. For pyrazole, a high ozone : pyrazole molar stoichiometry of 4.6 was found, suggesting that the transformation products contributed to the over-stoichiometric consumption of ozone (e.g., hydroxypyrazoles). Glyoxal and formate were the only identified transformation products (C mass balance of 65%). Overall, the identified major products are suspected to hydrolyze and/or be biodegraded and thereby abated by a biological post-treatment typically following ozonation. However, as substructures of more complex compounds (e.g., micropollutants), they might be more persistent during biological post-treatment.


 Please wait while we load your content...
Please wait while we load your content...