Investigation of metaldehyde removal by powdered activated carbon from different water samples†
Abstract
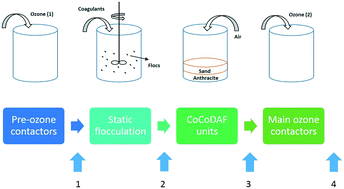
Metaldehyde as a widely used pesticide has been detected in surface water and drinking water in the UK with concentrations higher than the EU and UK standard (0.1 μg L−1). Previous studies have shown that powdered activated carbon (PAC) can adsorb metaldehyde even with the presence of natural organic matter, suggesting a promising solution to the problem. This paper studies the adsorption of metaldehyde onto PAC using different water samples including synthetic water, natural surface water, and water samples taken at different treatment processes from a water treatment plant. Metaldehyde (5 μg L−1) was effectively removed by PAC (50 mg L−1) from all water samples in this study, regardless of the water quality (74.3% to 99.7%). A PAC dosage of 100 mg L−1 was considered appropriate to remove metaldehyde at 5 μg L−1 after the first treatment process of pre-ozone treatment with a maximum adsorption capacity (qm) of 0.25 μg mg−1 given by the data fitted to the Langmuir isotherm model. Removal of metaldehyde by PAC was found to be most effective when PAC was applied after the static flocculation treatment process (98.4%) with a qm of 0.29 μg mg−1. The low adsorption capacity of PAC for low initial concentrations of metaldehyde solution was observed due to the lower driving force for mass transfer in the process of adsorption and competition with water molecules for adsorption sites on PAC.


 Please wait while we load your content...
Please wait while we load your content...