Organic structure and solid characteristics determine reactivity of phenolic compounds with synthetic and reclaimed manganese oxides†
Abstract
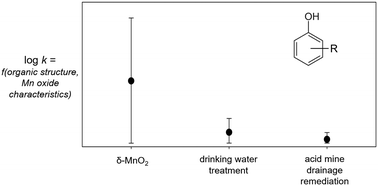
Manganese (Mn) oxides have been proposed for in situ treatment of organic (e.g., phenolic) contaminants, although little is known about the reactivity of reclaimed solids that might be used as alternatives to synthetic oxides. In this study, we investigate the impacts of phenol substituents and manganese oxide properties (e.g., surface area, iron substitution) on the kinetics and mechanism of this reaction. Reclaimed solids from acid mine drainage and drinking water treatment systems contain Mn(IV) and are capable of oxidizing phenolic contaminants, although their reactivity is 1–3 orders of magnitude slower than that of synthetic δ-MnO2. Both electron transfer-limited and sorption-limited mechanisms occur in 29 phenols reacted with the three manganese oxide materials. This finding contrasts with the common assumption that the first one-electron transfer from the phenol to the manganese oxide is rate-limiting. The occurrence of both mechanisms has implications for the rates and products of phenol oxidation. Interestingly, the mechanism for a given phenol changes between solids. We attribute this observed mechanism shift primarily to phenolic substituent effects, with influences from the pHpzc, surface area, and iron substitution of the manganese oxide materials. In addition, we investigate the predictive utility of quantitative structure–activity relationships, as these models have not been tested using complex reactants and non-synthetic manganese oxides. In-depth analysis and external validation measures indicate these common QSAR models are ineffective at predicting the behavior of complex contaminants or reactions with non-synthetic manganese oxides, and therefore have limited application for predicting contaminant oxidation by manganese oxides in environmental and engineered systems.


 Please wait while we load your content...
Please wait while we load your content...