Novel photocatalytic performance of nanocage-like MIL-125-NH2 induced by adsorption of phenolic pollutants†
Abstract
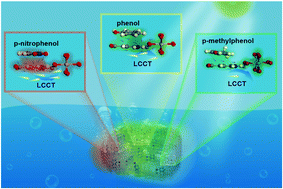
Adsorption–photocatalysis is a critical technique to meet the global challenges of environmental pollution and energy source shortages. However, scientific knowledge on how the adsorption process influences the subsequent photocatalytic performance is lacking. Here, we first propose a mechanism for understanding the intrinsic structure–performance relationship between phenolic pollutants and nanocage-like MIL-125-NH2 (NC-MIL-125-NH2) in adsorption–photocatalysis, from the perspective of electron induction. The experimental results, together with the density functional theory studies, soundly corroborate that the photocatalytic activity of NC-MIL-125-NH2 is enhanced and suppressed by the adsorption of electron-withdrawing pollutants i.e. p-nitrophenol and electron-donating pollutants i.e. p-methylphenol, respectively, which disagrees with conventional behavior. The mechanism is suitable to explain adsorption–photocatalysis behaviors when organic pollutants adsorb on the valence band of a photocatalytic material through π–π EDA interactions. These findings open new avenues for the design of functional materials for environmental and energy applications.


 Please wait while we load your content...
Please wait while we load your content...