Enhanced uptake of glyoxal at the acidic nanoparticle interface: implications for secondary organic aerosol formation†
Abstract
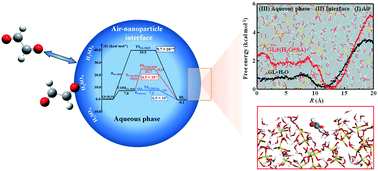
Glyoxal (GL) represents an important precursor of a secondary organic aerosol (SOA) and has broad biogenic and anthropogenic sources, but the interfacial chemistry of GL contributing to SOA formation is uncertain. Here, we performed molecular dynamics (MD) simulations and quantum chemical (QC) calculations to examine the interfacial process of gaseous GL on aqueous nanoparticles, including probing the GL attraction to the nanoparticles, uptake at the air–nanoparticle interface, and the hydration reaction within the nanoparticles under neutral and acidic conditions. The MD simulations show that the carbonyl O-atom of GL exhibits a preferential orientation to the air–nanoparticle interface. The acidic nanoparticle interface displays a preference for the uptake of gaseous GL relative to the neutral nanoparticle interface, showing that the presence of sulfuric acid (SA) enhances the adsorption and accommodation for GL. In addition, SA accelerates the aqueous reaction of GL inside the interior region of the nanoparticle, which indirectly promotes the uptake at the nanoparticle interface. Our results reveal the significantly enhanced uptake of GL at the acidic nanoparticle interface, highlighting the necessity to account for the interfacial process when assessing SOA formation.


 Please wait while we load your content...
Please wait while we load your content...