FeS colloids – formation and mobilization pathways in natural waters†
Abstract
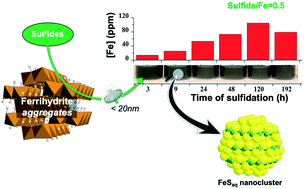
We have used synchrotron-based X-ray absorption spectroscopy (structure of Fe–S clusters), transmission electron microscopy (solid-phase crystallinity), Fourier-transform ion-cyclotron-resonance mass spectrometry (identity and composition of natural organic carbon compounds), inductively coupled plasma optical emission spectrometry (total aqueous Fe), and the revised ferrozine method (aqueous Fe(II) and Fe(III) concentrations) to determine the stability and nature of colloids generated by sulfidation of ferrihydrite nanoparticles in the absence and presence of organic compounds. We observed that reductive dissolution of ferrihydrite by aqueous sulfide generates nm-scale FeS clusters. Their subsequent aggregation, which promotes settling of FeS aggregates into the solid fraction, was directly correlated with sulfide/Fe ratio. At sulfide/Fe ratios ≤0.5, FeS clusters and larger colloids remained in suspension for at least 14 days (and up to several months). At sulfide/Fe ratios >0.5, sulfidation reaction rates were rapid and FeS cluster aggregation was accelerated. Moreover, the presence of organic compounds increased the time of suspension of FeS colloids, whereas increased ionic strength inhibited the generation of FeS colloids. We present a general conceptual model to predict when and where FeS colloids can form and enhance or inhibit the mobility of contaminants and nutrients associated with them. Our study indicates that in low-salinity fresh groundwater systems poor in sulfate (i.e. low sulfidation; lakes, floodplains, peatlands etc.), the ferrihydrite sulfidation reaction generates aqueous FeS clusters and larger colloids that remain suspended over long time periods, thus mobilizing a substantial fraction of the total aqueous Fe and S.


 Please wait while we load your content...
Please wait while we load your content...
