Stereoisomer specific reaction of hexabromocyclododecane with Fe(ii) associated with iron oxides†
Abstract
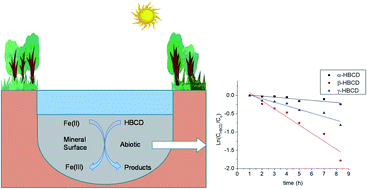
The reactions of hexabromocyclododecane (HBCD) isomers with Fe(II) associated with iron oxides were performed in a pH range from 6.15 to 7.50 at room temperature. It was observed that Fe(II) associated with iron oxides (i.e., goethite, magnetite, hematite) is a better reductant than just an aqueous solution of Fe(II) to potentially reduce HBCD in subsurface environments. The reaction of HBCD with Fe(II) associated with iron oxides is also stereoisomer specific with α-HBCD reacting much slower than β-HBCD and γ-HBCD. The reaction is pH dependent and it is faster with increased pH. The initial concentration of Fe(II) and HBCD can also affect the reaction rate. The reaction is negligible when all the Fe(II) is sorbed to magnetite and no Fe(II) remains dissolved. It was also observed that the reaction of 100 nM HBCD is slower than the reaction of 1.0 μM HBCD with Fe(II) associated with magnetite. In addition, natural organic matter (NOM) was found to inhibit the degradation of HBCD by Fe(II) associated with iron oxides.


 Please wait while we load your content...
Please wait while we load your content...