Photochemistry of 2,2-dichloroethanol: kinetics and mechanism of the reaction with Cl atoms and OH radicals
Abstract
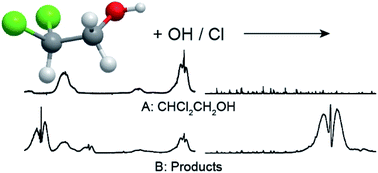
Smog chamber/FTIR techniques were used to investigate the kinetics and mechanism of the Cl atom and OH radical initiated oxidation of 2,2-dichloroethanol at (296 ± 1) K. Relative rate methods were used to measure k(Cl + CHCl2CH2OH) = (5.87 ± 0.96) × 10−12 and k(OH + CHCl2CH2OH) = (5.54 ± 1.94) × 10−13 cm3 molecule−1 s−1. Chlorine atom initiated oxidation of CHCl2CH2OH in one atmosphere of air gives HCOCl, CHCl2CHO, and COCl2 in yields of (62 ± 5)%, (39 ± 10)%, and (8 ± 2)%, respectively. The rate constant k(Cl + CHCl2CHO) = (8.3 ± 16) × 10−12 cm3 molecule−1 s−1 was determined and the IR spectra of CHCl2CHO is reported for the first time. The atmospheric lifetime for CHCl2CH2OH is estimated as 21 days. The experimental results are discussed in the context of the atmospheric chemistry of chlorinated alcohols.
- This article is part of the themed collection: Halogenated (semi)volatile organic compounds (“X(S)VOCs”)


 Please wait while we load your content...
Please wait while we load your content...