A simple field-based biodegradation test shows pH to be an inadequately controlled parameter in laboratory biodegradation testing†
Abstract
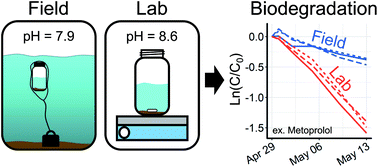
Biodegradation tests are essential for characterizing the behavior of organic micropollutants in the environment, but they are carried out almost exclusively in the laboratory. Test parameters such as temperature and test chemical concentration are often applied in ways that affect observed biodegradation, and laboratory testing requires sophisticated temperature-controlled facilities. We developed a field-based test based on OECD 309 which minimizes the need for laboratory resources such as temperature-controlled facilities by using bottles incubated in the natural water body. The test also utilized contaminant residues present in unspiked natural water to increase the relevance of the results to the local system. A test in a local river and a matching lab-based test were conducted in parallel. We quantified 26 of 40 targeted micropollutants and observed dissipation for 13. Significant differences in half-life (up to a factor of 3.5) between lab and field bottles were observed for 7 compounds, with 6 of 7 degrading more slowly in field bottles. For 4 of these, dissipation was positively correlated to the neutral fraction of the chemical. Differences in the neutral fraction arose due to a higher pH in the lab bottles induced by outgassing of CO2 from the oversaturated river water. We conclude that pH is an important parameter to control in biodegradation testing and that field-based tests may be more environmentally relevant.


 Please wait while we load your content...
Please wait while we load your content...