Thermal decomposition kinetics of glyphosate (GP) and its metabolite aminomethylphosphonic acid (AMPA)†
Abstract
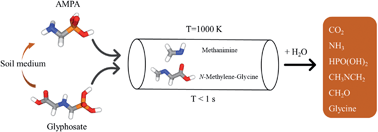
Glyphosate (GP) is a widely used herbicide worldwide, yet accumulation of GP and its main byproduct, aminomethylphosphonic acid (AMPA), in soil and water has raised concerns about its potential effects on human health. Thermal treatment, in which contaminants are vaporised and decomposed in the gas-phase, is one option for decontaminating material containing GP and AMPA, yet the thermal decomposition chemistry of these compounds remains poorly understood. Here, we have revealed the thermal decomposition mechanism of GP and AMPA in the gas phase by applying computational chemistry and reaction rate theory methods. The preferred decomposition channel for both substances involves the elimination of P(OH)3 to yield the imine N-methylene-glycine (from GP) or methanimine (from AMPA), with relatively low barrier heights (ca. 45 kcal mol−1). The half-life of GP and AMPA at 1000 K are predicted to be 0.1 and 4 ms respectively, and they should be readily destroyed via conventional incineration processes. The further decomposition of N-methylene-glycine is expected to also take place at similar temperatures, leading to N-methyl-methanimine + CO2, with a barrier height of ca. 48 kcal mol−1. The imine decomposition products of GP and AMPA are expected to react with water vapour to form simple amines and carbonyl compounds.


 Please wait while we load your content...
Please wait while we load your content...